How do Cells Decide Which Way to Grow? The Role of Invisible Mechanical Cues in Tissue Organisation
Human eye (left half) and Radial alignment of cells on a soft inhomogeneous substrate with an embedded glass bead at the centre (right half)
Image Credits: Michael Morse via Pexels and Dr Akshada Khadpekar. (Lead author of the study)
The following article was originally written by Manjeera Gowravaram and published here.
Researchers at IIT Bombay uncover how strain fields produced by embedded inhomogeneities within biomaterials influence cell alignment, reshaping our understanding of cell behaviour in development, disease, and tissue engineering.
Cells follow very specific patterns, whether in the human body or in engineered tissues. For instance, muscle fibres are aligned parallel to each other to enable coordinated movements, blood vessels extend toward wounds to facilitate healing, and cells in the eye are arranged radially to help focus light precisely onto the retina, ensuring clear and accurate vision. Such precise spatial organization is essential for proper tissue function. The arrangement of cells directly influences how effectively a tissue can carry out its role, be it contracting, transporting nutrients, or processing sensory input. But how do cells determine their correct location and orientation within these complex systems?
A new study from researchers at the Indian Institute of Technology Bombay, led by Prof. Abhijit Majumder, offers an answer: cells can sense and respond to invisible mechanical patterns—like built-in tensions around them. Their findings, published in Cell Reports Physical Science, not only adds to our fundamental understanding of how cells organize themselves, but also have important implications for tissue engineering, cancer research, and wound healing. The paper has been selected as part of a collection showcasing “some of the best biophysics research published in Cell Reports Physical Science”.
For decades, scientists believed that cells primarily relied on chemical signals, like growth factors or morphogens, to decide how and in which direction to grow. However, recent discoveries in this field suggest that mechanical signals are just as important. Cells can feel how stiff their surroundings are, detect tiny stretches, and even respond to surface textures smaller than themselves.
“In living tissue, mechanical inhomogeneities are common. You see it in tumours, healing wounds, developing organs. But we haven’t fully explored how cells interpret and respond to these physical cues,” says Prof. Majumder.
In their study, the researchers embedded a rigid object inside an otherwise soft material, mimicking mechanical inhomogeneity. The goal was to mimic how tissues naturally develop internal tension during processes like growth, injury, or tumour formation, and how cells might sense and respond to such forces.
“To simulate these conditions, we designed a soft polyacrylamide hydrogel with a small, rigid glass bead embedded inside. This setup replicates a rigid structure surrounded by softer material, like a tumour within body tissue,” explains lead author Dr. Akshada Khadpekar. When the gel was placed in water, it began to swell everywhere except where the bead was, because the stiff bead resisted the expansion. This created a pre-strain gradient— a varying stretch pattern around the bead.
When muscle precursor cells were added on the gel, the pre-strain gradient played a crucial role in guiding their alignment. “Cells near the bead detected the pre-strain gradient and aligned radially. As they exerted forces on the substrate, the mechanical signal propagated outward. This alignment extended about 1–2 mm (20–40 cell lengths) from the bead, reinforcing long-range organization,” says Dr. Khadpekar. On a soft, uniform gel without a bead, however, the alignment was limited to about 0.35 mm.
“Think of it like a shallow crater around the bead,” says Prof. Majumder. “But instead of falling in, the cells sense the stretching pre-strain and align accordingly.”
To be sure this wasn’t due to chemical factors, the researchers ran control experiments. They changed the type of extracellular matrix (ECM) proteins used to coat the gel and varied the stiffness of the substrate. Only on soft gels did the cells align. Harder gels masked the effect, and altering the ECM had no impact, proving that the alignment was not biochemical in origin.
To further investigate the mechanism behind cell alignment, the research team collaborated with Prof. Parag Tandaiya from the Department of Mechanical Engineering at IIT Bombay. The researchers employed finite element simulations to model the mechanical environment created by the swelling hydrogel. These computational models confirmed that the strain fields generated in the gel closely matched the patterns of cell alignment observed in experimental conditions.
“This was crucial because there’s no direct way to measure these subtle internal pre-strain fields experimentally. Without simulations, we wouldn’t have been able to formulate or test our hypothesis about what the cells were sensing,” says Prof. Tandaiya.
To test the generality of this phenomenon, the researchers extended their experiments beyond individual spherical beads to hollow glass capillary, glass beads and their combinations. In all cases, the cells aligned along invisible force patterns, forming arcs, waves, or spirals. The researchers also tested different types of cells and found that how the cells aligned depended on how much force they could apply and how stretched out they were. “The cells don’t just sense stretching of their substrate, they seem to also detect the direction in which the substrate is stretched the most and they line up in that direction. It’s a very precise and intelligent response, and we believe this is the first time such behavior has been observed in this way,” adds Prof. Tandaiya. Using these findings, a model was created to predict which cells would align based on their shape, strength, and the stiffness of the surface.
This discovery holds significant implications across multiple fields. “In tissue engineering, we might guide cell organization just by shaping soft materials, without complex scaffolds or stimulation. Or in cancer, the stiffness of tumours could explain how they influence nearby cells. And in regenerative medicine, adjusting tissue stiffness may help restore healthy cell patterns in aging or damaged tissues,” concludes Dr. Khadpekar.
**********************************************************
The original academic article titled Inhomogeneous Substrate Strain-Driven Long-Range Cellular Patterning was written by IIT Bombay’s researchers and published in the journal Cell Reports Physical Science.
The original paper can be found here.
Cracks in Blood Stains Could Tell Us How They Got There, According to a New Study
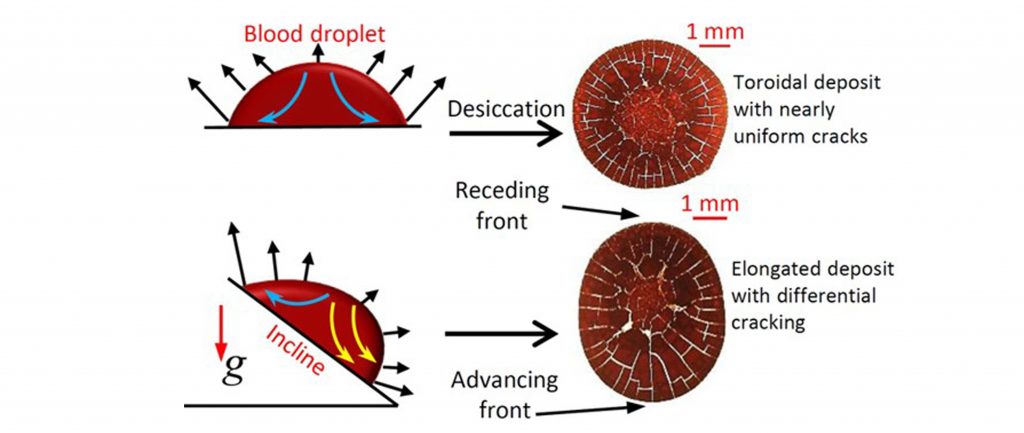
Graphical representation of an inclined plane affecting cracks in the dried blood droplet.
Image Credits: American Chemical Society (ACS)
The following article was originally written by Mr. Dennis Joy and published here.
Researchers at IIT Bombay studied how blood drops dried on tilted surfaces and what the cracks that are left behind can tell us about the blood drop.
The scene is often depicted in crime dramas: forensic scientists analyse bloodstains left behind, trying to piece together what happened. But beyond the dramatic flair, the science of how blood dries and the patterns it leaves is an area of research with implications far beyond the courtroom, extending into medical diagnostics and bioengineering. Picture a tiny drop of blood, no bigger than a raindrop, slowly drying on a surface. As the water evaporates, the solid material inside, mainly red blood cells, proteins, and salts, gets left behind, forming intricate patterns. Scientists call this desiccation, and the patterns aren’t random; they hold clues to how the blood got there in the first place, specifically, how much of it and the angle it fell.
Although desiccation patterns on flat surfaces have been studied since the early days of forensic sciences, there are still questions about what happens to a drop of blood when that surface is tilted. A team of researchers from the Indian Institute of Technology (IIT) Bombay tackled that question in a recent study exploring how tilting the surface changes how the blood dries and cracks.
The researchers wanted to understand the combined effect of how much blood is in the drop and how steep the surface is. They systematically tested different droplet volumes, from a tiny one-microliter drop the size of a pinhead up to 10 microliter drops the size of small buttons. The droplets were placed on glass surfaces tilted at various angles, from flat (0 degrees) to steep (70 degrees). They watched the drops dry in real time using high-speed cameras and then examined the dried patterns up close with microscopes and tools that measured surface height.
Drying blood drops on a flat surface typically form a ring-like deposit, much like the familiar coffee ring left by a spilt coffee drop. This happens because liquid evaporates fastest at the edge, pulling particles from the centre outwards. The dried blood ring, or corona, is dense with red blood cells and often shows cracks radiating outward.
But introduce a tilt, and gravity starts to pull the drop downhill, fighting against the surface tension that tries to keep it in a neat little dome. This battle deforms the drop, stretching it out and making the liquid surface uneven. The advancing edge, or the bottom half of the drop, tries to move downward, and the receding edge or the top half remains stationary. This leads to an asymmetric deposit, with the larger, heavier red blood cells affected more by gravity, settling towards the advancing side. The smaller components, like proteins and salts, are more influenced by the evaporation-driven flow, which is stronger where the contact angle is smaller, typically at the receding edge. So, on a tilted surface, we get a sorting effect with more red blood cells on the advancing side and smaller materials on the receding side.
This uneven distribution of material leads to striking differences in the cracks that form as the deposit dries completely. Cracking happens because the drying material shrinks, creating stress, like mud drying in the sun. When this stress gets too high, the material breaks, releasing the tension and creating new surfaces, in this case, the crack walls.
The researchers used a fundamental concept called the Griffith criterion, which says that cracking occurs when the energy released by the breaking material is enough to create the energy needed for the new crack surfaces. They developed a simple model based on this principle, considering the energy stored in the dried blood material and the energy required to create new surfaces at the interface between the blood and air, as well as the blood and the glass.
Their model showed that the stress needed to cause cracking depends on the thickness of the dried deposit. On the advancing side, where the dried blood mass accumulated more, the cracks were thicker and more widely spaced. The cracks were finer on the receding side, where the deposit thinned out. These differences are attributed to thicker and thinner deposit heights at advancing and receding fronts.
The cracking patterns are also asymmetric since the tilted surface causes an asymmetric deposit with different thicknesses on the advancing and receding fronts. At very steep angles and larger volumes, the drop might even slide a bit before settling, leaving a thin tail of dried material behind, which, being very thin, might not crack at all.
The study fills a crucial gap by exploring the factors influencing cracking and desiccation patterns. It systematically investigates the combined effects of volume and inclination and shows how these factors alter the resulting crack morphology. Moreover, their model establishes a relationship between the cracks that occur and the initial condition of the droplet before drying, which can be exploited for forensic analysis.
According to the study’s authors, cracks can tell us the initial droplet volume and impact angle. This finding can help to figure out the initial impact angle of the droplet, i.e., help in the reconstruction of crime in forensics.
While their model successfully explains the relationship between deposit thickness and cracking patterns qualitatively, the study has not explored the exact mechanism behind some observed features. The occasional and apparently random formation of a central bulge instead of central plaques in larger drops remains a subject for future investigation, highlighting a limitation in our current understanding of this complex process. The central bulge formation is not universal but occurs stochastically across different experimental runs. This could be attributed to flow instabilities during drying. The work, nevertheless, considerably improves our understanding of bloodstain patterns on different surfaces. Knowing how volume and surface angle influence these patterns in forensics can help investigators understand the blood splatters on non-flat surfaces.
Looking ahead, the team wants to study other differences in patterns. According to the authors, studies have shown subtle differences between the deposits of healthy and diseased persons’ blood. Different patterns as a function of the surface’s wettability are helpful from a forensics point of view. These could be possible biomarkers to study in the future.
**********************************************************
The original academic article titled Asymmetric Deposits and Crack Formation during Desiccation of a Blood Droplet on an Inclined Surface was written by IIT Bombay’s researchers and published in the journal Langmuir.
The original paper can be found here.
IIT Bombay Researchers Propose Bottling Sunlight in the Summers to Use as
Heating in the Winter

Image Courtesy: Pexels
The following article was originally written by Mr. Ankush Banerjee and published here.
Scientists Are Using Chemistry to Trap Summer Sun for Freezing Winters
For nearly a quarter of the year, temperatures in the cold Himalayan north plunge well below freezing. To keep warm, families in places like snow-covered Leh rely on diesel heaters, which are carbon-heavy machines powered by costly fuel that must be trucked in over perilous winter roads. But what if the same intense summer sun that bathes these mountains could be captured and saved for later, ready to warm homes months after it has set? A team of researchers from the Indian Institute of Technology Bombay (IIT Bombay) believes this is possible through the power of chemistry.
In a recent study, the team proposes using thermochemical storage powered by a compound called strontium bromide to address the region’s seasonal heating needs. Strontium bromide has been the subject of many studies examining thermochemical storage. Much like a battery stores electricity, thermochemical storage stores heat in the form of chemical energy. Strontium bromide is chosen for its high energy density, chemical stability, non-toxicity, non-explosiveness, and environmental safety.
The researchers developed a prototype that first uses solar thermal air collectors which harness sunlight to heat air in the summer. Next, this hot air is used to warm a form of hydrated strontium bromide (hexahydrate). In this form, the strontium bromide crystals contain water molecules within their structure. During this heating process, the material undergoes an endothermic dehydration reaction, in which heat energy is absorbed, causing the expulsion of water molecules from its crystal structure. This reaction stores the absorbed solar energy as chemical potential in the resulting monohydrate salt. In winter, moist air is passed through the “charged” salt, triggering a reverse reaction or rehydration. The process reintroduces the water molecules into the structure in an exothermic reaction, releasing the stored heat.
The technology is a sustainable solution in Himalayan regions, which face long, frigid winters. The population has limited access to sustainable heating options and usually depends on diesel or firewood. Therefore, what makes thermochemical storage particularly suited to these regions is its ability to retain energy over several months.
Author Dr. Rudrodip Majumdar, who worked on this project as a postdoctoral fellow at IIT Bombay and is now working at the National Institute of Advanced Studies (NIAS), shared that this technology is as personal as it is academic. “I went to the Chopta-Tungnath-Chandrashila trek and stayed in camps. The starry nights in the Himalayas are beautiful, but the night stay can turn very hostile,” he explains. “I’ve seen people struggling, walking ten kilometers just to collect firewood. Diesel becomes the only backup, but it’s a heavy polluter.”
To help the communities, a module capable of storing about 500 kilowatt-hours of energy was designed. This is enough energy to heat a small Himalayan home for up to four months, as per the team. The whole setup consists of a simple, modular unit designed to be easy to transport and operate. It includes solar thermal collectors that heat air during summer, a reactor chamber filled with strontium bromide salt, and a small air circulation system to trigger the dehydration and rehydration cycles. The reactor components are housed in a compact, weatherproof unit designed for Himalayan conditions and are insulated using glass wool.
“Solar collectors are well-proven. Steel tanks have been made for ages. The only new contribution is stabilising the thermochemical material and packaging it for daily life. This kind of long-term seasonal storage is made possible because the energy stored in the material is very stable. It does not degrade over time,” added Dr. Majumdar.
The modest module doesn’t take up the entire room, either. According to Dr. Sandip Kumar Saha from IIT Bombay, who led the study, “Each storage module is roughly the size of two LPG cylinders and designed to be portable. They can be recharged in the summer at solar stations—possibly even in sunnier regions like Gujarat or Rajasthan—and trucked up to Himalayan towns just before winter.”
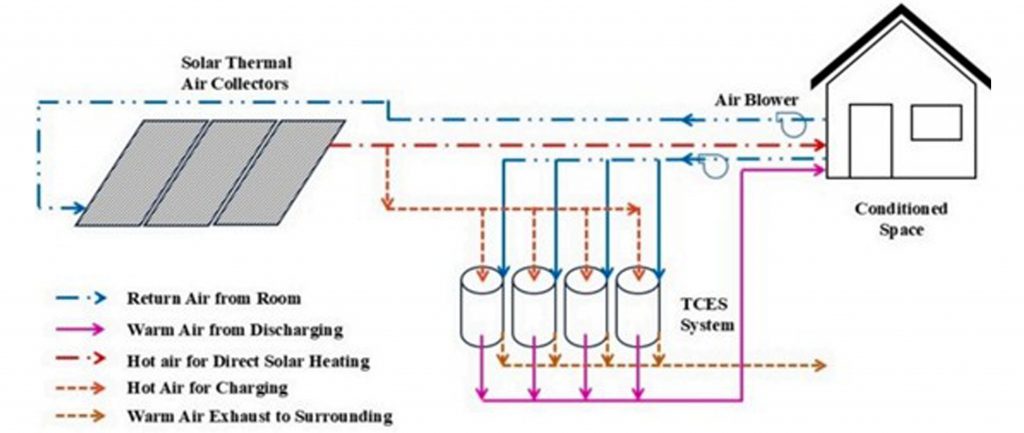
Schematic representation of the proposed seasonal solar heating system and the airflow (A.S. Pujari et al., Applied Thermal Engineering 269 (2025) 126090)
“Once you deploy this material, you don’t need to change it,” adds Dr. Majumdar. “If you maintain the reactor with basic precautions, the operation and maintenance costs come down. These are very sturdy reactors.”
The researchers have already tested the material through six full charging and discharging cycles in the lab, with no performance degradation. Thermochemical salts like strontium bromide are theoretically capable of 500 to 600 cycles, the researchers explain, meaning that each unit could last years.
While the upfront investment may be higher than diesel heaters, the study finds that thermochemical systems are more economical over time—especially in remote regions where diesel prices are inflated due to transportation costs and where the environmental impact, or potential penalties from carbon emissions, adds to the overall burden.
“If we want to produce electricity from diesel today, it will cost us ₹50 per unit (kWh),” said Dr. Majumdar. “If we add a carbon penalty, it could go up to ₹78 per unit. Then the thermochemical solution will be half the price.”
The study calculated the thermochemical systems’ Levelized Cost of Heating (LCOH), the average cost of producing usable heat over the lifetime of a heating system, to be between ₹33–₹51 per kWh in different Himalayan cities. This cost makes it competitive with or cheaper than diesel heating for daily use, especially when factoring in fuel transport and environmental costs. In Leh specifically, LCOH dropped to ₹31/kWh, the lowest among all locations (Darjeeling, Shillong, Dehradun, Shimla, Jammu, Srinagar, Manali, and Leh) studied.
“Such solar-thermal energy solutions for space-heating have been successfully tested in harsh climatic conditions for the Indian army at sub-zero temperatures. We are in the process of developing thermal storage solutions for round-the-year, smoke-free heating to camps of our Indian army stationed at such high altitudes,” adds Dr. Chandramouli Subramaniam from IIT Bombay, who worked on the study.
Despite the promising findings, the system still faces hurdles before it can be scaled for real-world use. Internationally, thermochemical storage has only been piloted in places like Germany, with limited large-scale adoption. In India, practical deployment in homes is yet to be tested, and initial costs remain relatively high compared to diesel heaters. The system also relies on adequate summer sunlight to “charge” the salt and sufficient winter humidity to release the stored heat—factors that vary across Himalayan regions. Still, the researchers believe that with field trials, policy support, and local engagement, the technology can become a crucial part of a more sustainable and inclusive energy future.
“Energy is very related to quality of life,” Dr. Majumdar notes. “Energy poverty should not exist in the 21st century. We need to ensure that even the remotest parts of the country are energy secure.”
**********************************************************
The original academic article titled Techno-Economic Analysis Of Solar Thermal Seasonal Thermochemical Storage For Indian Himalayan Cities was written by IIT Bombay’s researchers and published in the journal Applied Thermal Engineering.
The original paper can be found here.

