1 – A Novel IIT Bombay “Paddle-Wheels” Sensor to Detect Heavy Metals in Water
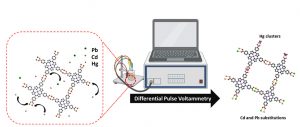
Graphical representation of Cu-TCPP sensor detection of Cd, Pb and Hg atoms.
Image Credit: Prashanth Kannan
The following article was originally written by Mr. Dennis C. Joy and published here.
The low-cost sensor made of a copper-based metal-organic framework performs as well as DNA based sensor, the gold standard for water quality sensors.
Heavy metals are elements with high atomic weights and densities. They play significant roles in various sectors, from manufacturing to agriculture. However, despite their utility, heavy metals also pose significant environmental and health concerns due to their potential toxicity, persistence, and bioaccumulative (ability to accumulate within living organisms) nature.
According to a report by The Energy and Resources Institute (TERI), nearly 718 Indian districts have groundwater contaminated with heavy metals, such as arsenic, cadmium, chromium, and lead. The Ministry of Environment, Forest and Climate Change (MoEF&CC) has also identified 320 locations as having a high probability of contamination with heavy metals. Ingesting these metals can cause serious health problems, including damage to the skin, bones, brain and other organs, especially in children. Efficient detection of these metals in water is crucial for ensuring environmental safety and public health.
In a bid to address heavy metal pollution, researchers from the Indian Institute of Technology, Bombay, and Monash University, Australia, with funding support from the Department of Biotechnology (DBT), Govt. of India, have developed a sensor using a copper-based metal-organic framework (MOF) to detect toxic metals in water more cost-effectively and efficiently.
Metal-organic frameworks (MOFs) are a class of materials characterised by their highly porous structures. At a microscopic level, these frameworks are composed of nodes of metal ions connected by organic compounds, forming a porous network with tunable properties and immense surface area to volume ratio. Due to their unique structure and versatility, MOFs have garnered significant interest in various scientific and industrial applications.
For their study, the team of researchers fabricated an MOF with copper (Cu) forming the metal nodes connected by the organic compound Tetrakis (4-carboxyphenyl) porphyrin, forming copper-tetracarboxyphenylporphyrin, or Cu-TCPP for short. The Cu-TCPP is a two-dimensional (2D) MOF with a paddle-wheel structure. The unique structure also means Cu-TCPP can have more surface area contacting the water and is much more efficient at picking up heavy metal ions than conventional 3D materials. The sensor is able to detect heavy metal ions like lead (Pb), cadmium (Cd) and mercury (Hg) in water samples, even when there are only a few atoms per millilitre present.
“This MOF involves two Cu atoms binding to each carboxyphenyl arm of the TCPP molecule, hence forming the characteristic paddle-wheel structure. This means that other metal ions with similar configurations would be able to replace Cu in the structure and maintain the overall order without causing structural collapse. Other metal ions, especially heavy metal ions, can also accumulate on the MOF lattice,” explains Prashanth Kannan, the first author of the paper and a student at the IIT Bombay-Monash Research Academy, talking about the structure of the Cu-TCPP MOF.
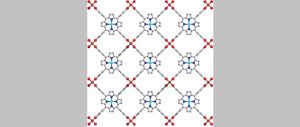
Paddle-wheel structure of the Cu-TCPP MOF, with red copper atoms binding to the white TCPP molecule.
Image Credit: Authors
The Cu-TCPP detects heavy metal ions in water in two ways – by substitution, where a metal ion knocks the copper out and replaces it, or by accumulation, where the metal ions just accumulate on the surface. Lead has incomplete p-orbitals, meaning it needs more electrons to be stable. This incompleteness allows the lead to replace the Cu ions in the MOF seamlessly while still allowing the MOF to maintain its structure. Once the lead replaces the copper, MOF’s electronic properties also change, which allows researchers to measure the amount of lead in the water.
Metals like cadmium and mercury, on the other hand, don’t easily substitute with copper ions. Instead of replacing the copper, these metals accumulate at the surface, forming what are known as molecular islands on the surfaces of metals. “When faced with a highly regular periodic lattice arrangement such as the Cu-TCPP MOF, they initially accumulate on the surface of the MOF, then at high concentrations can cause the failure of the MOF structure. By identifying the differences in electrochemical waveform and intensity (during the failure), we are able to accurately estimate nanomolar levels of heavy metals in water,” explains Prashanth.
The researchers tested the sensor on water samples from taps and lakes. It accurately detected the three metals, lead, cadmium and mercury, even when present in trace amounts. The sensor performed well despite being tested with substances that could interfere with the MOF, like alkali metals, debris and other large particles, in the water, indicating its reliability in different conditions. The researchers then also compared their device with the state-of-the-art sensors available in the market and found that it performed comparably, if not better, in most cases. “Our device has the least complexity and comparable sensing limits to the best of the current DNA-based sensors (the gold standard for sensing devices),” remarks Prashanth.
Despite its performance, the sensor does have limitations. After a single use, the MOF structure tends to break down upon prolonged exposure to heavy metals. This means the sensor can only be used once. However, in the particular case of water quality sensors, since the industry standard for low-cost devices is one-time use, reusability is not needed and is not really a limitation of the sensor, according to Prashanth. “The major bottleneck with this type of device lies in material fabrication costs. MOFs are difficult to coat over large areas, but there are ongoing efforts by various research groups worldwide to make manufacturing possible on a large scale,” he adds.
The technology not only holds promise for improving public health but also highlights the potential of science to develop solutions to pressing environmental challenges. Prashanth is already looking ahead to the next challenges. “Currently, there are several topics of major interest worldwide that need materials like MOFs to address them, like detecting Perfluorooctane sulfonic acid (PFOS), perfluoroalkyl substances (PFAS), arsenic and chromium in drinking water and tap water,” he signs off indicating future applications.
**********************************************************
The original academic article titled Tripartite Detection and Sensing of Toxic Heavy Metals Using a Copper-Based Porphyrin Metal–Organic Framework was written by IIT Bombay’s researchers and published in the journal, ACS Applied Materials & Interfaces, Vol 16/Issue 46.
The original paper can be found here.
2. Study Suggests Strategies to Extract Truth from Unwilling Senders
Image Credit: Wikimedia Commons
The following article was originally written by Ms. Arati Halbe and published here.
Offering limited options to choose from for a multiple choice setting recovers more accurate and truthful information than presenting the complete range of options, reveals IIT Bombay study.
One would recall, with pain and much irritation, the times when one had to travel internationally during the COVID-19 times. If you happened to be the one inside the city receiving passengers, you would be afraid; what if the incoming passengers travelled through places with higher infection? If you were the traveller, you would like to believe that you are not infected and would want to avoid reporting your travel through COVID-19-affected cities. Health officers, on the other hand, had a tough situation to deal with. They had to extract as much truth from people unwilling to disclose the whole truth. All they could do was ask questions and believe the answers were true.
If you are wondering if there is any chance of recovering the truth, you can stop worrying! In a first of its kind study, Dr Anuj Vora and Prof Ankur Kulkarni from the Indian Institute of Technology Bombay (IIT Bombay) tackle the challenge of how the receiver can design the right questions to learn as much truth as possible when the sender is not entirely cooperative, and there could be errors in communication due to noise.
Problems related to extracting information from people who are unwilling to disclose information or non-cooperative senders occur frequently, say, during negotiations. Negotiating parties may not give truthful information because they think that disclosing some facts may lead to an unfavourable deal. Extraction of information from non-cooperative senders is studied extensively in mechanism design theory. Roger Myerson laid the foundations of mechanism design theory and was awarded the 2007 Nobel Memorial Prize in Economic Sciences, along with Leonid Hurwicz and Eric Maskin.
“Mechanism design has not attempted to quantify the amount of information obtainable in settings where all information may not be obtainable,” explains Prof Ankur Kulkarni. In situations like the one faced by the health officer in COVID-19 times, it may not be possible to retrieve the complete travel history of all the travellers. However, it is important to know how much information can be obtained. “Quantification of information is the subject of information theory. We are the first to perform an information theoretic analysis of a problem that is broadly within the domain of mechanism design,” says Prof Kulkarni.
The current study shows that despite the sender being non-cooperative and the communication being noisy, the receiver can still recover a huge number of possible correct answers. At the same time, there are a huge number of correct answers that cannot be recovered, however cleverly the questionnaire is designed.
“Our results show on the one hand how a receiver may strategise to obtain information from such agents, and on the other that there will usually be blind spots in the knowledge of the receiver, regardless of how it strategises,” say the researchers.
Vora and Kulkarni quantify the amount of information that can be extracted by defining a quantity called the ‘information extraction capacity’. They established a method to calculate the range of values (the upper and lower limits) for this quantity and show that in several cases, the information extraction capacity can be exactly calculated. Their study provides strategies that the receiver can use to design the questionnaire and also a structural understanding of the kind of information that can be recovered.
The study assumes that the receiver will ask just one question and present a list of possible answers as options from which the sender chooses one correct answer. For example, a health officer may present sequences of cities visited before arriving at the current port. In a naive approach, the officer would list all possible sequences as the choices. However, it turns out that this approach gives travellers more opportunities to lie if they have information to hide. If the options are limited, travellers who wish to disclose some travel sectors but hide others will tend to report more truthfully. The officers can recover the most truth by keeping the number of options within the optimal range, as suggested by the study.
The health officer may wish to know only a few cities visited previously. Thus, the length of the ‘sequence’ of cities may be limited to just a handful. However, in another situation, say when tax officers are trying to trace a chain of financial transactions, the sequence they wish to recover will be longer. More choices will need to be offered for the multiple-choice questions the officers ask. The number of optimal choices to be offered will grow with the increasing length of the sequence to be recovered. The researchers define the rate of growth of the number of optimal choices as the information extraction capacity. The quantity of communication resources required when communicating with a non-cooperative sender depends on the information extraction capacity.
Vora and Kulkarni bring in an aspect from information theory and model the communication when the communication itself may be noisy or not very accurate. For example, if one is trying to send a message over a communication line and say the line changes the letter B to D each time, then if the receiver gets D, they will assume it is D even when B is sent, making communication ambiguous for two letters (B and D). What it means in this case is that only 24 letters out of 26 can be sent without error. The amount of information that can be sent without errors is termed the zero-error capacity of the channel. In the current study, Vora and Kulkarni established that to utilise the information extraction capacity of the sender, the zero-error capacity of the channel needs to be more than the information extraction capacity and the receiver can extract a huge number of sequences in this case.
The study offers an insight into the basis of why certain questionnaires, such as in immigration or options offered by customer care bots, are not exhaustive. As users, we may frequently need to select an option that closely matches our case when we do not find an exact match. “Our study demonstrates that providing limited options in multiple choice questions may not be due to bad design, but it may be a strategy crafted to obtain as much truthful information as possible from a large number of users,” comments Prof Kulkarni.
This research was supported by the grant from the Science and Engineering Research Board, Department of Science and Technology, India.
The finding has applications in different fields, including finance, control systems, intelligence gathering and national security, market research and diplomatic negotiations. “Our results in this paper provide not only strategies for the receiver, but also a structural understanding of the type information that can potentially be recovered,” concludes Prof Kulkarni.
**********************************************************
The original academic article titled Shannon Meets Myerson: Information Extraction from a Strategic Sender was written by IIT Bombay’s researchers and published in the journal, Mathematical Social Sciences, Volume 131.
The original paper can be found here.
3. Coastal Vegetation to Mitigate Tsunami and Coastal Flood Impacts

Image Credits: Pxhere
The following article was originally written by Ms. Divyapriya Chandrasekaran and published here.
A sustainable and resilient method to reduce wave forces and debris impact during extreme tsunami and coastal flood events
It’s been two decades since the devastating tsunami struck the Indian Ocean, which left an indelible mark on the minds and lives of thousands of people. The destruction and devastation could have been more if not for the myriad natural barriers along India’s coastline – its mangroves. This natural catastrophic event highlighted the need for an effective measure to reduce the tsunami wave force and trapping debris. Several ‘storm surge’ events – cyclone-induced flooding events as the sea rises – occur every year. Coastal mangroves act as bio-shields against such disasters. The traditional method of constructing sea walls is possible, but they are expensive and may disrupt natural processes.
Researchers from the Indian Institute of Technology Bombay (IIT Bombay) focused on evaluating how emergent coastal vegetation acts as a natural barrier against tsunami impacts. In their recent study, the researchers used both experimental and numerical methods to investigate the effectiveness of mangroves in reducing tsunami-induced debris impacts on buildings and bridges. They created a smoothed particle hydrodynamics (SPH) model, a computational method used to simulate the flow/behavior of fluids, to observe complex interactions between water, vegetation, and debris.
“We need to understand that Nature is supreme and we must align and work with nature and not against. In this case, waves, coastal currents and coastal sediment transport are the predominant natural processes. It is advisable that any coastal defence system must not adversely interfere with the natural processes,” remarked Prof. Behera, from the Department of Civil Engineering at IIT Bombay, about the need for natural barriers.
Among the varied vegetation types found in the coastal regions, researchers have chosen the emergent vegetation type for the study. Emergent vegetation is aquatic plants rooted in the soil, while their stems, leaves, and flowers emerge above the water surface. Mangroves are emergent trees with sturdy submerged roots, stiff stems, and trunks to reduce wave forces. “Mangroves are the best examples of natural bio-shields against extreme ocean disasters. The mangroves present at Bhitarkanika, Odisha have safeguarded the coastal regions against cyclones that attack almost every year,” says Prof. Behera. On the contrary, the study found that floating and submerged vegetation types are either swept away by tsunami waves or not strong enough to dissipate the wave energy.
The experimental set-up involved a replica of a coastal region using a large water tank (dam-break flume) containing a scaled-down column and an aluminium debris model. The column structure mimicked a coastal building, and the debris model was a replica of a shipping container. A vertical sliding gate was opened to release high-speed water mimicking tsunami-like conditions in the tank. Upon releasing the water, the sensor in the column measured the impact force of the debris hitting the structure. Similarly, the accelerometer in the debris model recorded its speed and movement before impact. The study found that heavier debris causes more impact forces on the column structure.
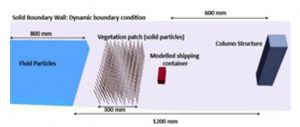
Simulated Coastal Defence System
Image Credits: Dr Aditya Gupta’s PhD thesis at IITB-Monash Academy, IIT Bombay (supervised by Prof Behera).
The numerical method involved computer simulations to measure the performance of the vegetation. The SPH modelling was used to simulate the debris impact on the column structure and the effectiveness of the vegetation in decreasing the wave forces. This simulation studied the wave interaction on the models of two types of emergent vegetation—rigid staggered vegetation (RSV) and tilting staggered vegetation (TSV). RSV stays upright, which represents the rigid mangroves or stiff emergent vegetation in real scenarios, while TSV symbolizes a natural bend in vegetation due to forceful waves.
The SPH simulation tested the performance of vegetation in reducing the wave force, slowing down the debris movement, and lowering the wave height, using three indices—Reduced Fluid Force Index (RFI), Reduced Momentum Index (RMI), and Transmission Coefficient (CT), respectively. RFI and RMI are higher for rigid staggered vegetation than tilting staggered vegetation. The rigid vegetation efficiently resisted enormous volumes of water and reduced the wave energy. Compared to the 89% reduction of debris impact with tilted vegetation, the rigid vegetation reduced the debris impact on the column structure by 96%.
“The rigid emergent vegetation can be planted along the coastal zones to reduce erosion, provide protection against storm surges and coastal floodings. Vegetations also known as bio-shields are the eco-friendly protection that will act as carbon sinks and help to achieve net zero target of India,” adds Prof. Behera.
The study shows that the emergent type of vegetation is an effective defence system that significantly reduces the damage caused by tsunami waves to coastal infrastructure. Further study is required to replicate it in natural conditions with varied vegetation types, patterns of wave movement, and different types of debris materials. Researchers believe that future studies can focus on advanced simulations for more accurate findings.
The findings of this research provide clues to coastal planners on how to select and use vegetation types in designing a better disaster mitigation strategy. This encourages policymakers and engineers to adopt a resilient, cost-effective, and sustainable defence system, fostering the coastal ecosystem using a nature-based solution.
**********************************************************
The original academic article titled Effectiveness of Emergent Coastal Vegetation as a Defense System to Mitigate Debris Load on a Structure During Extreme Events was written by IIT Bombay’s researchers and published in the Ocean Science Journal.
The original paper can be found here.
4. Cobalt-Based Catalysts And More: Reducing Carbon Emissions In Steel Industry

Image Credit: Wikimedia Commons
The following article was originally written by Manjeera Gowravaram and published here.
Combining hydrogen-based processes with advanced catalysts and renewable energy paves the way for developing economically and industrially viable solutions to decarbonise the steel industry
Globally, steel is a vital component of modern infrastructure and economic progress. India is among the top producers of steel. However, steel production is closely linked to environmental concerns as it relies heavily on coal as a fuel. In the steel production process, carbon (primarily sourced from coal and natural gas) reacts with iron ore to produce molten iron, which is then refined to create steel. However, this process also generates vast amounts of carbon dioxide (CO₂) but it also generates vast amounts of carbon dioxide (CO2). As a result, the steel industry globally emits over 3.7 billion metric tons of CO2 every year, contributing to 7–9% of carbon emissions. Steel production can be made sustainable by adopting a method called hydrogen-based direct reduction of iron (H-DRI).
In a recent review published in the Journal of Energy and Climate Change, researchers from the Chemistry Department at the Indian Institute of Technology Bombay (IIT Bombay), led by Prof. Arnab Dutta, have collated the advances made in the field of hydrogen generation for the steel industry and put forward the best way to decarbonise the steel industry using ‘green’ hydrogen.
The H-DRI process uses hydrogen to convert iron ore into steel instead of coal, releasing water vapour rather than carbon dioxide as a byproduct during the manufacturing process. This makes hydrogen a great option for “decarbonising” the steel industry. Currently, most of the hydrogen comes from processes like steam methane reforming or coal gasification. Both rely on fossil fuels, which still generate CO2, defeating the primary purpose.
So, to produce hydrogen sustainably, researchers are shifting towards water electrolysis — a process of splitting water into hydrogen and oxygen using electricity in an electrolyser device. If renewable energy sources like wind or solar can power the electricity, the process becomes emission-free, hence the term ‘green hydrogen’. However, producing green hydrogen at an industrial scale is expensive as it needs considerable infrastructure modifications and effective catalysts.
Catalysts are essential to make the water electrolysis used for hydrogen production effective. Generally, noble metals such as platinum and palladium are used as catalysts. “These (noble metals) are expensive and limit large-scale applications, and are not suitable for harsh or remote conditions,” says Dr. Suhana Karim, a postdoctoral research fellow in Prof. Dutta’s lab. “So, the focus is on finding alternatives that are economically viable and sustainable,” she adds.
Researchers worldwide, including Prof. Dutta’s group, are developing cobalt-based catalysts (cobaloximes) that are water soluble and air-stable to aid electrolysis without requiring specialised equipment. Cobaloximes are cheaper than noble metals and can be synthesised easily.
Several researchers have improved the stability and reaction rates of cobaloximes by modifying their molecular structure. For example, Prof. Dutta and his team have added natural amino acids, vitamins, and other functional groups into the catalyst’s structure to increase hydrogen production rates while maintaining energy efficiency. “We have also modified cobaloximes to work effectively in the presence of various minerals and salts, such as in seawater,” adds Dr. Suhana.
Cobaloximes work well in labs, but it is complex to use them for industrial hydrogen production. Hence, researchers are modifying their structure to make it compatible with the electrodes of the electrolyser and attaching them to solid supports to enhance stability, efficiency, and durability.
The researchers also analysed different types of electrolysers and furnaces to improve hydrogen production using renewable energy for industry. They found that cobaloxime catalysts perform well in both alkaline electrolysers, using solutions like potassium hydroxide, and proton exchange membrane electrolysers, which use a solid polymer membrane in acidic conditions.
“Each type has strengths and weaknesses in cost, durability, and efficiency,” explains Prof. Dutta.

Prototype of a Multi-Stack Electrolyzer Developed by Prof. Arnab Dutta’s Research Group.
Image Credits: Arnab Dutta And Suhana Karim
Researchers from IIT Bombay also point out that the traditional blast furnace-basic oxygen furnace method uses a lot of coal to produce steel, releasing a significant amount of CO₂. In contrast, the electric arc furnace uses electricity, and when powered by renewable energy, it produces less carbon emissions. Researchers believe that by combining hydrogen-based direct reduction of iron with electric arc furnace technology, steelmaking can become nearly carbon-neutral.
The use of green hydrogen can further be combined with carbon capture, utilisation, and storage (CCUS) strategies to further reduce emissions. CCUS systems capture any leftover CO₂ from steelmaking or other processes, allowing its use to produce synthetic fuels or chemicals or stored deep underground for the long term. This approach also promotes a circular economy by reusing CO₂ in productive ways.
The IIT Bombay study highlights how water electrolysis using cobalt-based catalysts, and
choosing suitable electrolysers and furnace types, can produce green hydrogen-based steel. This approach helps significantly reduce carbon emissions, paving the way for a cleaner and more sustainable future for steel production.
**********************************************************
The original academic article titled Cobalt-Based Molecular Electrocatalyst-Mediated Green Hydrogen Generation: A Potential Pathway For Decarbonising Steel Industry was written by IIT Bombay’s researchers and published in the journal Energy and Climate Change, Volume 5.
The original paper can be found here.

